Interim Recommendations for the New COVID-19 (2023-2024 Formula) Vaccines
Summary
This document is for educational purposes and is intended only to assist with application setup. It is not intended to provide clinical guidance. Users of Net Health software and providers are responsible for reviewing all vaccination guidance provided by the CDC prior to administering any vaccines
Please use this link,CDC Updated (2023-2024 Formula) COVID-19 Vaccine, to access the recommendations for administering the 2023-2024 Formula.
Please make sure to check often as these recommendations can change. We are providing an interim document for your use.
This documentation is from the information that has been updated on the CDC website on 9/22/23.
It is our understanding that the older COVID-19 vaccines will be discontinued.
Vaccine Names, CVX, MVX, NDC, and CPT Codes
| Product Name | MVX Code | CVX Code | CVX Short Description | UoS NDC10 UoS NDC11 | UoU NDC10 UoU NDC11 | Packaging | CPT Code |
|---|---|---|---|---|---|---|---|
| COMIRNATY (COVID-19 Vaccine, mRNA, 2023-2024 Formula) | Pfizer (PFR) | 309 | COVID-19, mRNA, LNP-S, PF, tris-sucrose, 30 mcg/0.3 mL | 0069-2362-10 00069-2362-10 | 0069-2362-01 00069-2362-01 | CARTON, 10 SINGLE-DOSE VIALS | 91320 |
| COMIRNATY (COVID-19 Vaccine, mRNA, 2023-2024 Formula) | Pfizer (PFR) | 309 | COVID-19, mRNA, LNP-S, PF, tris-sucrose, 30 mcg/0.3 mL | 0069-2392-10 00069-2392-10 | 0069-2392-01 00069-2392-01 | CARTON, 10 PRE-FILLED SYRINGES | 91320 |
| Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) | Pfizer (PFR) | 310 | COVID-19, mRNA, LNP-S, PF, tris-sucrose, 10 mcg/0.3 mL | 59267-4331-2 59267-4331-02 | 59267-4331-1 59267-4331-01 | CARTON, 10 SINGLE-DOSE VIALS | 91319 |
| Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) | Pfizer (PFR) | 308 | COVID-19, mRNA, LNP-S, PF, tris-sucrose, 3 mcg/0.3 mL | 59267-4315-2 59267-4315-02 | 59267-4315-1 59267-4315-01 | CARTON, 10 MULTI-DOSE VIALS | 91318 |
| Novavax COVID-19 Vaccine, Adjuvanted | Novavax (NVX) | 313 | COVID-19, subunit, rS-nanoparticle, adjuvanted, PF, 5 mcg/0.5 mL | 80631-105-02 80631-0105-02 | 80631-105-01 80631-0105-01 | CARTON, 2 MULTI-DOSE VIALS | 91304 |
| Spikevax | Moderna (MOD) | 312 | COVID-19, mRNA, LNP-S, PF, 50 mcg/0.5 mL | 80777-102-95 80777-0102-95 | 80777-102-04 80777-0102-04 | CARTON, 10 SINGLE-DOSE VIALS | 91322 |
| Spikevax | Moderna (MOD) | 312 | COVID-19, mRNA, LNP-S, PF, 50 mcg/0.5 mL | 80777-102-96 80777-0102-96 | 80777-102-01 80777-0102-01 | CARTON, 10 PRE-FILLED SYRINGES | 91322 |
| Spikevax | Moderna (MOD) | 312 | COVID-19, mRNA, LNP-S, PF, 50 mcg/0.5 mL | 80777-102-93 80777-0102-93 | 80777-102-01 80777-0102-01 | CARTON, 10 BLISTER-SEALED PRE-FILLED SYRINGES | 91322 |
| Moderna COVID-19 Vaccine | Moderna (MOD) | 311 | COVID-19, mRNA, LNP-S, PF, 25 mcg/0.25 mL | 80777-287-92 80777-0287-92 | 80777-287-07 80777-0287-07 | CARTON, 10 SINGLE-DOSE VIALS | 91321 |
|
UoS (Unit of Shipment) is the lot number for the vaccine packaging UoU (Unit of Use) is the lot number for the vial. To view this information select this link Fall 2023 Covide-19 Codes and Crosswalk |
|||||||
Adding Medical Activities, CVX, and CPT Codes to Your Application
Adding the CPT Codes
-
From the Home Nav bar
-
Select Setup/Admin
-
Select Medical Codes
-
Select CPT-4 / HCPCS Codes
-
The CPT or HCPCS Codes window opens
-
From the bottom of the window select Add
-
The Enter CPT or HCPS Codes (New) opens
-
Enter the new CPT code information
-
Complete the General and Billing tabs
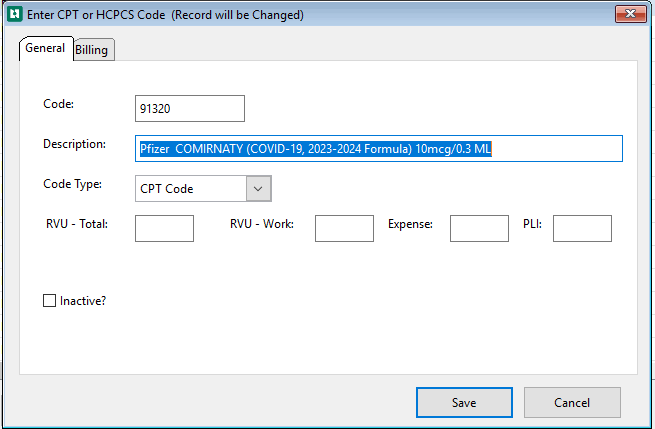
-
Select Save
Adding a new Medical Activity Code
When adding your medical activity make sure to include the CVX code. This is required if using the Mobile Immunization Tracking application. If these are not added the import will not map correctly.
-
From the Home Nav bar
-
Select Setup/Admin
-
Select Medical Codes
-
Select Medical Activities
-
The Select Medical Activity window opens
-
From the bottom of the window select Add
-
The Enter Medical Activity Code (New) window opens
-
Enter the Activity Code and Description
-
Add the CVX Code
If using MIT the CVX code must be entered on your Medical Activity. This insures that the vaccine selected in MIT will import to the correct Medical Activity in your application.
-
Enter the rest of the required information on the General tab
At this time the CDC is not recommending any additions doses be given for anyone 5 to 11 years and 12 years and older. See the CDC guide lines for dosage information by age.
Use these links COVID-19 (2023-2024 Formula) Vaccination Recomendations and COVID-19 (2023-2024 Formula) Immunization Schedule Ages 6 Months to 12 Years and Older
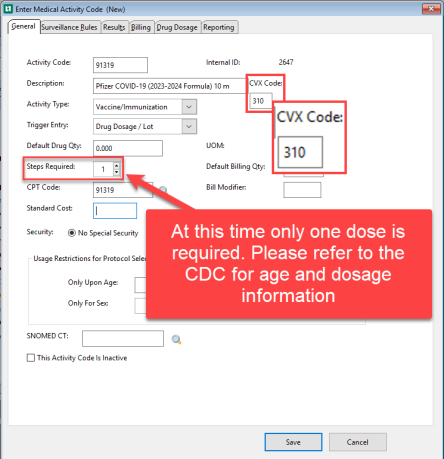
-
Complete the rest of the tabs and select Save.
It is our recommendation that the activity be set as a one step with no repeat.